Stay Ahead of the Curve With Our Innovative Regulatory Software Solutions
At the heart of Freyr Digital’s suite of Regulatory software prowess lies the knowledge we have gathered over decade.
In a world overflowing with data, we navigate with wisdom, carve out new paths with creativity, and drive forward with the most advanced tech solutions.
Know More
Freyr Digital Helps...
Centralize Your Regulatory Data
Say goodbye to scattered data and documents. Our Regulatory Information Management (RIM) solutions offer a centralized platform for all your Regulatory data and documents. Manage submissions, track communications with Regulatory authorities, and ensure compliance, all in one place.

Freyr Digital Helps...
Streamline Your eCTD Submissions
Streamlining submissions has never been easier. With our electronic Common Technical Document (eCTD) software, you can effortlessly assemble and submit your Regulatory paperwork electronically, minimize manual errors, maintain adherence to electronic submission standards, and enhance the efficiency of your submission workflow.
Freyr Digital Helps...
Optimize Document Management
From creation to archiving, manage all your Regulatory documents efficiently with our Document Management System (rDMS) solutions. Improve document traceability, ensure compliance with document control requirements, and reduce manual efforts.

Freyr Digital Helps...
Unlock Strategic Decision Making With AI-Powered Conversations
Experience unparalleled efficiency in gathering Regulatory information with Freyr Digital AI innovations. Our suite of AI tools including an intuitive chatbot & intelligent summarizer is engineered to collect and simplify complex Regulatory data, enabling you to seamlessly make informed decisions.

Freyr Digital Helps...
Streamline Your Label & Artwork Creation With Collaborative Workflows
Effortlessly enhance efficiency, minimize errors, and streamline collaborative multi-user workflows for artwork and label management using our cutting-edge software.

Accelerate & Empower.
Drive Your Strategic Goals Forward.
With Productivity, Efficiency, and Compliance.
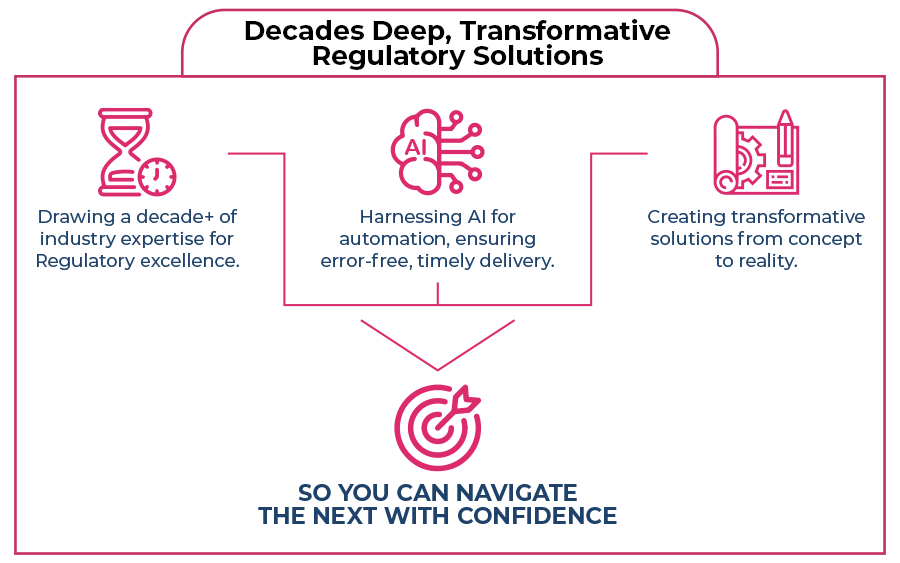
How We Do It?
 10+ years of hands-on experience.
10+ years of hands-on experience. Aligning tech prowess with Regulatory expertise.
Aligning tech prowess with Regulatory expertise. Putting your goals in the forefront of every solution we develop.
Putting your goals in the forefront of every solution we develop.
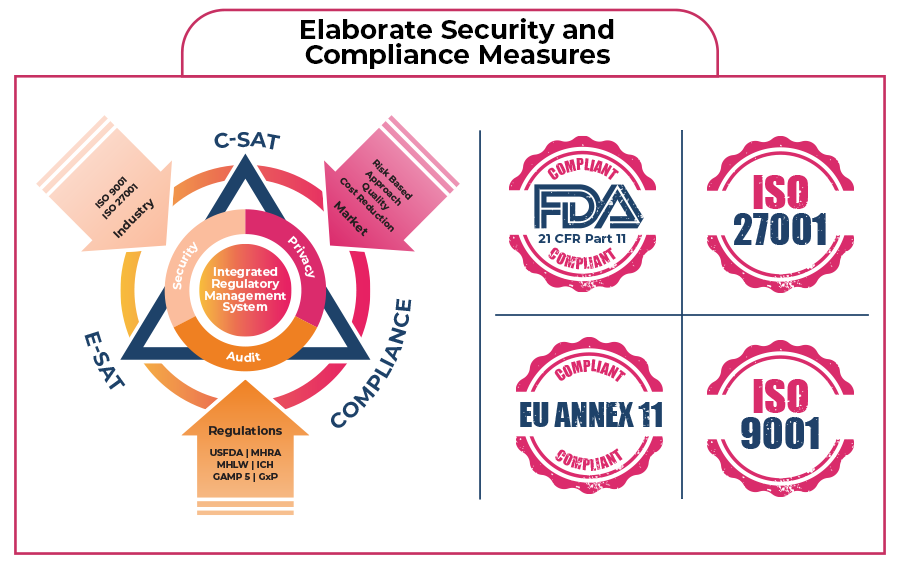
Key Features of All Our Products

Save almost 57% of publishing efforts! The cutting-edge eCTD submission software not only streamlines but also speeds up your Regulatory submission process.
Top Features
- Inbuilt eCTD Validator & Viewer
- Supports All Major eCTD Templates
- On-Premise & Cloud Deployment

An electronic Regulatory Document Management System that offers a centralized platform for easy document management and collaboration.
Top Features
- Version Control
- Role Based Access
- Advanced Search Functionality

Stay fully compliant by managing and tracking all Regulatory submission documents, commitments and correspondence with our advanced RIM solution.
Top Features
- Complete Registration Lifecycle Management
- IDMP Readiness
- Tailored Reports and User Dashboards

AI enabled platform that gathers Regulatory information from numerous Health Authorities and other websites.
Top Features
- Global Regulatory Updates
- Global Regulatory Requirements
- 100+ countries Regulations Tracked in Near Real-Time

Stay compliant with changing cosmetic industry regulations effortlessly. Freyr iREADY gathers ingredient and Regulatory information for cosmetics storing updates from global sources and tracking 122,000+ cosmetic ingredients and 490,000+ chemicals.
Top Features
- Recent Updates
- Ingredient Database
- Formulae Compliance Checker

Robust platform to create, validate, store, and submit complex content structures aligning with SPL (FDA) and SPM (HC) standards.
Top Features
- Interactive Dashboards & Workflow Management
- Automation & Validation
- Text Comparison

Simplify, create, review and approve artwork in collaborative environment saving up to 70% of your time.
Top Features
- Collaboration Between Stakeholders
- Customized Workflows & Forms
- Digital Asset Library
Join our growing community of customers and embrace the digital way forward.
Join our growing community of customers and embrace the digital way forward.
Join our growing community of customers and embrace the digital way forward.
Join our growing community of customers and embrace the digital way forward.
Partner Up
As part of Freyr Digital's PartnerUp program, you'll join a supportive community that is dedicated to helping each other succeed. We offer our partners the resources and support they need to deliver exceptional results for their clients and grow their businesses.