Imagine working on a new drug to treat a devastating disease. After years of hard work, you've finally gathered the data you need to submit a regulatory submission to the FDA. But then, you realize that your electronic records and eSignatures don't comply with 21 CFR Part 11. This is a scenario that no one wants to find themselves in. The world of regulatory compliance is complex and ever evolving. With new regulations being introduced all the time, it can be difficult for businesses to keep up. However, one regulation that is essential for any business that operates in a regulated industry: 21 CFR Part 11.
21 CFR Part 11 is a regulation that establishes the criteria for electronic records and eSignatures in FDA-regulated industries. It is designed to ensure that electronic records are trustworthy, reliable, and equivalent to paper records and handwritten signatures. 21 CFR Part 11 is relevant for regulatory submissions because it provides guidance on ensuring that electronic records and eSignatures comply with FDA requirements. It is important because regulatory submissions often contain a significant amount of electronic data, such as clinical trial data, manufacturing records, and quality control data.
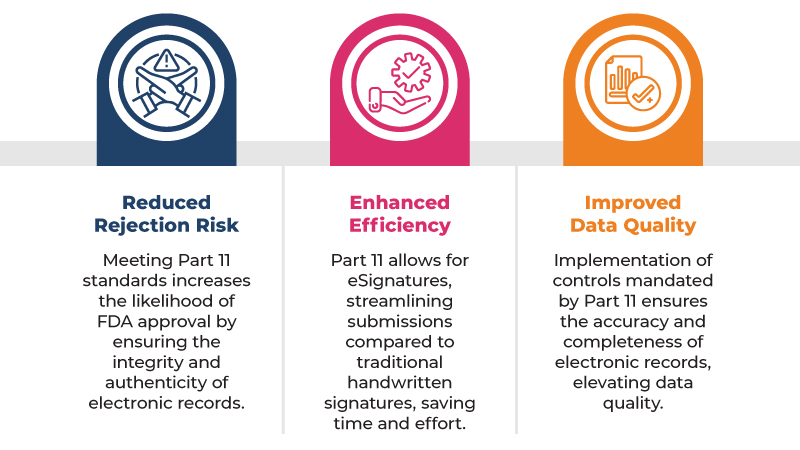
Here are some of the key benefits of complying with 21 CFR Part 11 for regulatory submissions:
To comply with 21 CFR Part 11, companies must implement a number of controls, including:
- Validate Systems: Ensure electronic systems meet Part 11 requirements, including robust security, audit trails, and data integrity testing.
- Control User Access: Restrict access to authorized personnel through password protection, electronic signatures, and other authentication methods.
- Maintain Audit Trails: Implement audit trails to track all record changes, enabling the detection and investigation of unauthorized alterations.
- Ensure Data Security: Employ data security measures to safeguard electronic records from unauthorized access, use, disclosure, disruption, modification, or destruction.
By complying with 21 CFR Part 11, companies can help to ensure that their electronic clinical trial data is reliable and trustworthy. This is important because FDA inspectors are more likely to approve regulatory submissions that contain high-quality electronic clinical trial data.
21 CFR Part 11 compliance is integral to a successful submission to the FDA. And, with a submission software like Freyr SUBMIT PRO which centralizes management, enhances collaboration, and ensures swift eCTD submissions and is 21 CFR Part 11 compliant you can achieve this. To know more request a demo now!
