
Introduction
The life science regulatory landscape is undergoing a profound transformation. As companies strive to bring life-saving innovations to market faster, the traditional approaches to regulatory management are proving increasingly cumbersome, inefficient, and prone to error. Fragmented systems, siloed data, and manual processes not only slow down operations but also introduce significant compliance risks. At Freyr, we recognize that the future of regulatory affairs demands a paradigm shift – a move towards platforms that are not just functional, but inherently intelligent, adaptable, and collaborative.
Our platform, Freya Fusion, embodies this future, built around a powerful mantra: Composable, Connected, and Cognitive. These three pillars are the foundational qualities of next-generation regulatory systems, designed to address the inherent complexities of our industry and unlock unprecedented levels of efficiency, compliance, and innovation. This blog post delves into how these principles are setting a new standard for regulatory excellence.
Explore freya fusion’s unified AI-RIMS
Understanding the Challenges of Existing Approaches
The regulatory functions within life science companies have been characterized by an "Existing Approach" marked by significant hurdles, as illustrated in the current industry view:
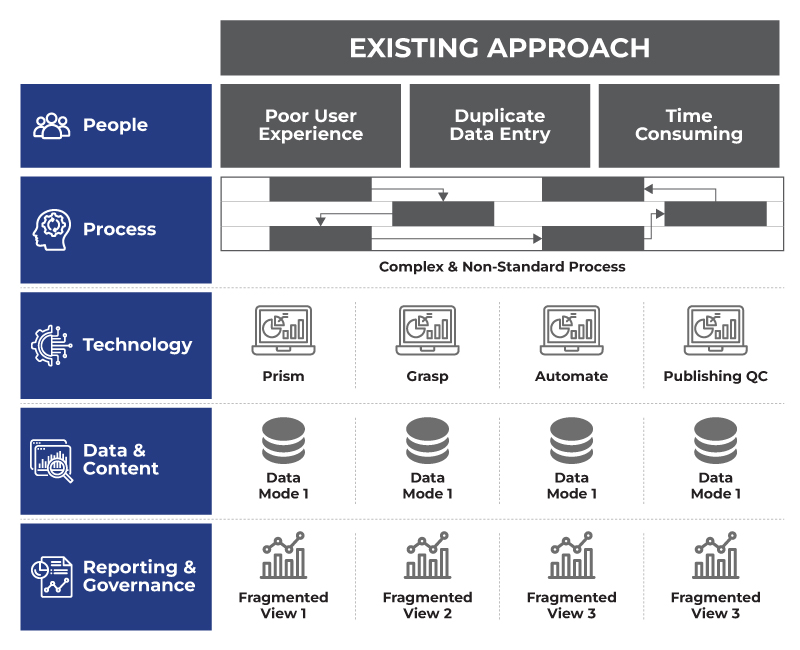
- People: Suffer from a poor user experience and time-consuming tasks due to duplicative data entry. This leads to frustration and drains valuable resources that could be better spent on strategic initiatives.
- Process: Are often complex & non-standard, with disjointed workflows that create bottlenecks and inefficiencies. The lack of a unified flow makes it difficult to track progress and ensure consistency.
- Technology: Relies on disparate systems, each handling specific tasks in isolation. This creates technological silos that hinder seamless operations.
- Data: Is trapped in different, disconnected databases leading to fragmented data view and making data reconciliation a constant challenge.
- Reporting & Governance: Result in fragmented views making comprehensive oversight nearly impossible. This lack of clear visibility impedes informed decision-making and proactive risk management.
These challenges underscore the urgent need for a unified regulatory platform that can overcome these limitations and propel the industry forward.
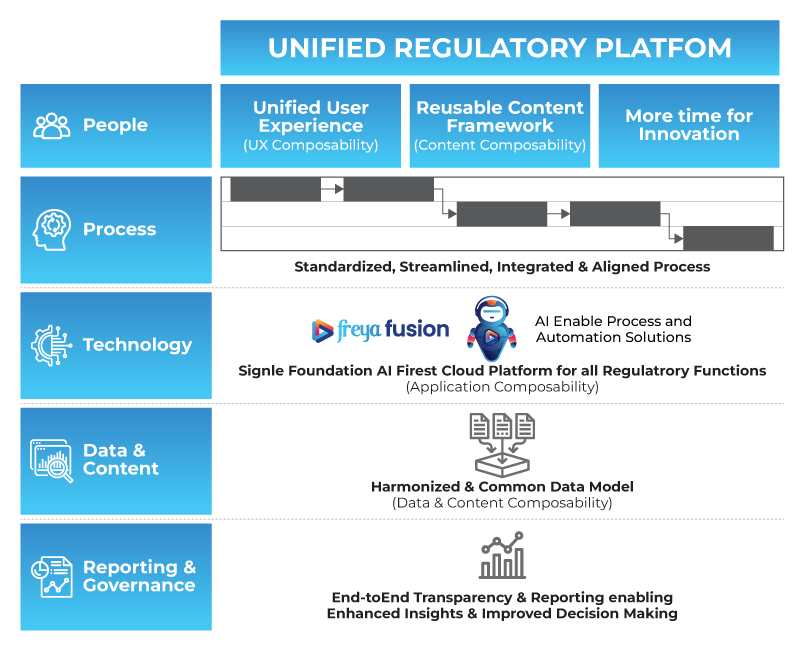
Enter freya fusion, a unified AI-First Regulatory Information Management System designed end to end for regulatory operations, including label management, document control, intelligence, automation, and conversational Q&A. With its modular architecture, freya fusion helps organizations solve people, process, technology, data, reporting and governance challenges.
Composable: Building a Flexible and Adaptable Regulatory Ecosystem
In a rapidly evolving regulatory landscape, agility is paramount. This is where the power of Composable platforms comes into play. A composable regulatory platform is inherently modular and flexible, built on a microservices architecture. This means instead of a monolithic, rigid system, organizations can "compose" exactly the capabilities they need, when they need them.
Consider the flexibility this offers: a company might initially focus on implementing a robust Regulatory Information Management (RIM) system. As their needs evolve, they can seamlessly add a structured content authoring module, or integrate new functionalities like a global labeling solution, without having to rebuild their entire system. This composability allows for rapid scaling and customization, enabling businesses to adapt quickly to new regulations, market demands, or internal process changes. It's about empowering users to configure their ideal regulatory environment, ensuring that the platform grows and evolves with the business, rather than becoming an outdated constraint. This modularity is key to achieving application composability, supporting a future where solutions are tailored, not forced.
Connected: Unifying Regulatory Functions for a Single Source of Truth
Imagine a regulatory world where every piece of information, every document, and every process is seamlessly linked. This is the essence of a Connected regulatory platform. Traditionally, critical regulatory functions—from document management and submission tracking to labeling and regulatory intelligence—operated in isolated silos. This meant redundant data entry, inconsistent information, and a constant struggle to gain a holistic view of regulatory activities.
A connected platform fundamentally breaks down these data silos and links processes end-to-end. By consolidating disparate systems and data models into a harmonized and common data model, it yields a true single source of truth. This unified environment fosters smoother collaboration across teams, departments, and even external partners. For instance, a change in a labeling document instantly propagates through the submission system, ensuring all linked elements are updated. This interconnectedness not only eliminates manual errors and duplicative efforts but also provides the end-to-end transparency crucial for enhanced insights and improved decision-making, as envisioned by the unified regulatory platform approach.
Cognitive: Infusing Intelligence for Proactive Compliance Management
The ultimate leap forward in regulatory platforms lies in their Cognitive capabilities – the embedding of Artificial Intelligence (AI) and machine learning (ML) to provide actionable intelligence and drive automation. This transforms regulatory operations from reactive to predictive and proactive.
A cognitive platform leverages vast amounts of regulatory data to deliver unprecedented insights. For example:
- An AI can auto-classify documents, accurately tagging and organizing incoming regulatory intelligence or internal content, drastically reducing manual effort and improving searchability.
- Through learning from past approvals and submission outcomes, AI can suggest optimal submission strategies, identifying the most efficient pathways to market and minimizing potential roadblocks.
- Chatbot answers complex regulatory questions instantly, drawing from a comprehensive knowledge base and real-time regulatory updates, empowering users with immediate, accurate information.
These cognitive capabilities turn raw data into strategic insights, enabling predictive compliance management. By anticipating potential issues, identifying trends, and automating routine tasks, cognitive platforms like Freya Fusion empower regulatory teams to move beyond administrative burdens and focus on higher-value activities, ensuring not just compliance, but also competitive advantage. This AI-enabled approach is the core of our "Single Foundational AI First Cloud Platform for all Regulatory Functions," driving true automation and intelligent decision support.
Conclusion: The New Standard for Regulatory Excellence is Here
The future of life science regulatory affairs is no longer a distant vision; it's a tangible reality built on the pillars of Composable, Connected, and Cognitive platforms. By moving away from fragmented, complex existing approaches, and embracing a unified regulatory platform, companies can achieve:
- Unified User Experience leading to improved productivity and satisfaction.
- Standardized, Streamlined, Integrated & Aligned Processes for unparalleled efficiency.
- AI-Enabled Process and Automation Solutions for intelligent operations.
- A Harmonized & Common Data Model ensuring a single source of truth and data integrity.
- End-to-End Transparency & Reporting for enhanced insights and improved decision-making.
