
Life sciences is a highly regulated industry, full of sensitive information that is required to be maintained efficiently. Documents are the backbone of any life sciences segment since it establishes the quality of processes and assurance of safety. The growing volume of documents in organizations exponentially increases the demand to replace traditional and manual documentation practices to reduce time, effort, and errors. In such scenarios, the absence of a proper Document Management System (DMS) increases the chance of misplacing critical documents and tracking changes significantly.
The major challenges in managing multiple documents include the following:
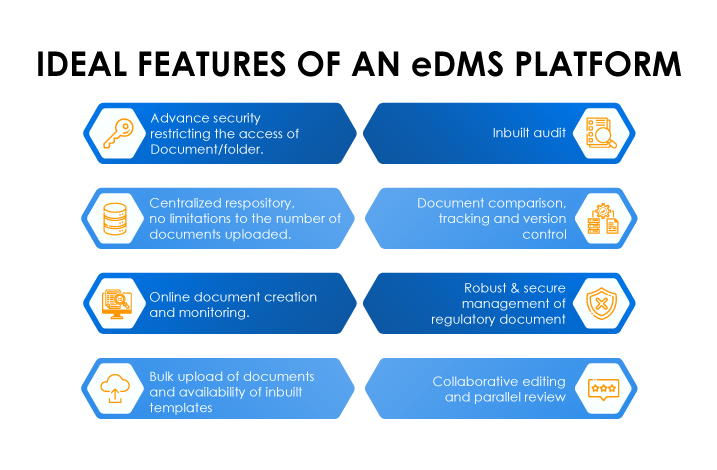
- Limited Visibility to the Content
- Lack of Audit Trails
- Need for Collaboration
- Need for Change Tracking and Version Control
- Time-taking Document Archival
- Unrestricted but controlled access to important documents
A smart and secure electronic Document Management System(eDMS) can provide precision and control for complex product development. Documents from diverse functional areas can be accessed easily without extra work or duplication, thanks to comprehensive content and data repositories. A robust content repository can help handle the data from all functional areas, including the previously developed documentation.
The Evident Gains of Having a Well-developed eDMS:
- It helps to maintain the consistency of data and avoid data redundancy
- Supports the whole document lifecycle from inception and authoring up to submission and archiving of Regulatory documents
- eDMS plays a vital role in managing submissions documents collectively in one place for improved submission readiness
- It ensures document security and authenticity, and it is compliant with 21 CFR part 11, which will reap the benefits of an organization for paperless record-keeping systems
- It supports the compilation of folder structures in eCTD format as per the standards of the FDA or local country formats to an equivalent authority
- Improves timeliness, document authoring, and collection
- It facilitates advanced admin functions to manage users and monitor ground-level activity
- Ensures compliance with global HAs by an effective organization of documents coupled with an efficient document monitoring process
- Efficiently classifies and stores all essential documents with reliable backups
An eDMS enables teams to collaborate and get an outlook on global Regulatory operations. Freyr rDMS, an end-to-end electronic Regulatory Document Management System (rDMS/eDMS), is exclusively designed to enable Regulatory groups and departments within a life sciences organization to effortlessly create, capture, manage, organize, connect, deliver, and archive Regulatory data and documents. The solution is built from the ground up, cognizant of Regulatory strategies and operational functions.
Reach out to Freyr experts to understand electronic document management system. Click here to go through our proven software – Freyr eDMS/rDMS. Request a demo.
