
In today's highly regulated business landscape, organizations face the formidable challenge of managing an ever-growing volume of documents to ensure Regulatory compliance with diverse standards and regulations. To navigate through complexities such as document overload, compliance costs, increased audit scrutiny, etc., many businesses are adopting Regulatory Document Management Systems (RDMS). This blog explores how RDMS can streamline compliance efforts, improve productivity, and reduce risks, ultimately driving organizational efficiency.
Streamlining Compliance Efforts
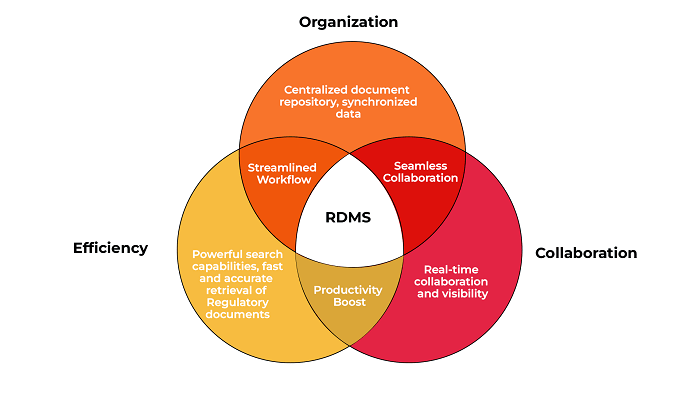
Centralized Document Repository: RDMS provides a centralized document repository for all Regulatory documents, eliminating the need for multiple systems or scattered file storage. This consolidation improves accessibility and ensures the availability of the latest document versions to authorized personnel.
Document Control and Collaboration: RDMS establishes standardized workflows, approval processes, and document change management, streamlining collaboration among stakeholders and ensuring compliance throughout the document lifecycle.
Automated Reminders and Notifications: RDMS automates reminders and notifications for document reviews, updates, and expirations, ensuring timely compliance activities and reducing the risk of missed deadlines.
Improved Productivity and Efficiency
Easy Document Retrieval: RDMS offers powerful search capabilities, enabling quick and accurate retrieval of Regulatory documents based on keywords, metadata, document types, or Regulatory requirements. This saves time and enhances productivity by eliminating manual searches through extensive document repositories.
Workflow Automation: RDMS automates document-centric workflows, reducing manual effort and improving efficiency. Automated approval processes, document routing, and notifications facilitate seamless collaboration and faster turnaround times.
Integration with Enterprise Systems: RDMS integration with other enterprise systems such as ERP, CRM, or QMS optimizes efficiency. Synchronized data between systems eliminates duplicate data entry, enhances data accuracy, and ensures consistent compliance across the organization.
Risk Mitigation and Auditing
Audit Trail and Version Control: RDMS maintains a comprehensive audit trail, tracking all document-related activities, including changes, approvals, and access history. This feature provides transparency, accountability, and traceability, facilitating internal and external audits and compliance assessments.
Document Security and Access Control: RDMS implements robust security measures to protect sensitive Regulatory documents from unauthorized access or tampering. Role-based management access control ensures that only authorized personnel can view, edit, or approve documents, minimizing the risk of data breaches or compliance violations.
Compliance Reporting: RDMS generates accurate and up-to-date compliance reports, simplifying the reporting process for Regulatory agencies. Real-time visibility into compliance status, expirations, and gaps enable proactive decision-making and timely remediation.
A Regulatory Document Management System (RDMS) empowers organizations to effectively manage Regulatory documents, streamline compliance efforts, and enhance overall efficiency. An RDMS mitigates risks, improves productivity, and establishes a solid foundation for successful Regulatory compliance by centralizing document storage, automating workflows, and ensuring data security.
Freyr rDMS, an end-to-end Regulatory Document Management System (rDMS/eDMS) that enables organizations to stay ahead in today's dynamic business environment, bolstering their reputation and driving sustainable growth.
Contact Us to know more.
