RIMS stand for Regulatory Information Management System and is a centralized software platform that simplifies Regulatory industry's product application and registration lifecycle tracking. It enables end-to-end tracking of Regulatory activities related to different kinds of products like Pharmaceuticals, Biologics and Cosmetics, etc.
Why is RIMS important?
Over time, submission management process in Regulatory space has become multifaceted and challenging. A robust RIMS system can help organize the work throughout the lifespan of the product development and medicine marketing. RIMS improves Regulatory business planning and organizes the Product Registration process to track and manage all Regulatory activities and life cycle. It also avoids duplicated effort and streamlines product applications and submission operations. In brief, RIMS came into existence to avoid redundancy and manual effort.
Competencies of RIMS
- Reduces effort and timelines to obtain Regulatory compliance concerning products and Regulatory organizations
- Delivers clear oversight of lifecycle compliances
- Effectively manages the post-submission queries raised by Health Authorities
- Helps manage commitments, variations, and obligations easily
- Tracks marketing authorization and lifecycle applications
- Tracks dossiers for variation in products and Regulatory organizations
- Tracks variations, reviews of records, and product information
- Accomplishes Regulatory mandates of electronic systems, such as 21 CFR Part 11
- Improves efficiency and collaboration with increased speed and quality of the submission
How does RIMS function?
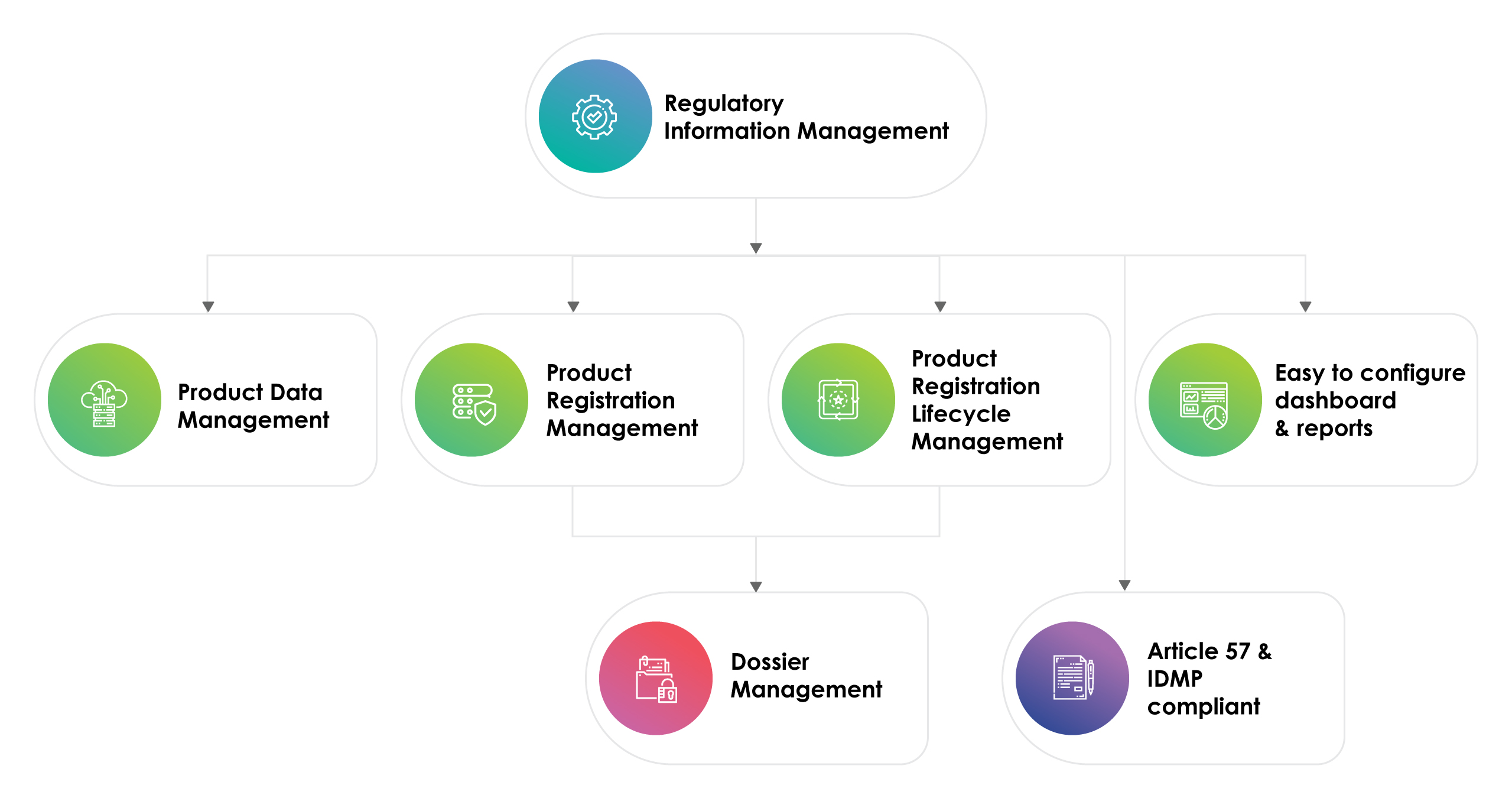
RIMS is a combination of document and data management solutions with product registration and lifecycle process tracking to accelerate Regulatory development across the globe. The tool predominantly supports the end-to-end Regulatory data management. It basically consists of: -
- Product data management
- Product registration management
- Product registration lifecycle management
- Dossier management
- Article 57 & IDMP compliant
- Easy to configure dashboard & reports
The user-friendly nature of RIMS interface eliminates the complexities of product submissions and streamlines procedural activities. RIMS eliminate manual tracking errors and reduces the risk for tracking global data, and eases submissions.
Freyr Digital has created a unique RIMS landscape to ease the challenges faced by the industry. To know more about Freyr RIMS, reach out to us - hello@freyrdigital.com.
